Hepatotoxicity
HemoGenix® is a Compliant Contract Research Service Supplier through Scientist.com
![]()
For more information on hepatotoxicity texting, please contact HemoGenix® at contractresearch@hemogenix.com or call (719) 264-6250.
Types of Hepatotoxicity Studies Available
The majority of studies performed by HemoGenix® incorporate a Complete Service, Full Report that can be used for an IND application. More recently, Sponsors have requested a more streamlined study and studies involving high-throughput screening. For this reason, HemoGenix® now provides 3 types of study format.
- Complete Service, Full Report Study: Fully customized study that includes the Study Plan, Draft Text and Final Text Report with QA audit.
- Rapid Toxicity Study: A customized study that includes the Study Plan and full protocol, raw results and graphical data in a single Excel Workbook. No formal text report and no QA audit is performed. This type of study is designed for early drug development. No interpretation or conclusions are provided.
- High-Throughput Screening Module (presently only available for human studies): A screening study is designed for high-throughput screening of compounds during ADME/Tox screening in multiples of 5 on specific cell populations to provide the most important ranking information. The full protocol, raw results and graphical data are provided in an Excel Workbook. No interpretation or conclusions are provided. This type of Screening Study is part of the ComparaTOX™ HT Platform.
Drug-Induced Liver Toxicity (DILI)
Hepatotoxicity comes in 2 main forms, namely "intrinsic" and "idiosyncratic". Whereas intrinsic drug-induced liver (DILI) toxicity is predictable and can affect all patients at some dose, idiosyncratic drug-induced liver toxicity usually only affects certain individuals and is rarely, if at all, predictable.
Dose-dependent, intrinsic, DILI can be detected and measured using the HepatoGlo™-Tox HT Platform. This high-throughput, multi-species, standardized and validated ATP bioluminescence assay can be used for different sources of hepatocytes as well as cell lines, such as Hep G2, and can be multiplexed with CYP 450 assays.
Hepatocyte Sources for HepatoGlo™-Tox HT
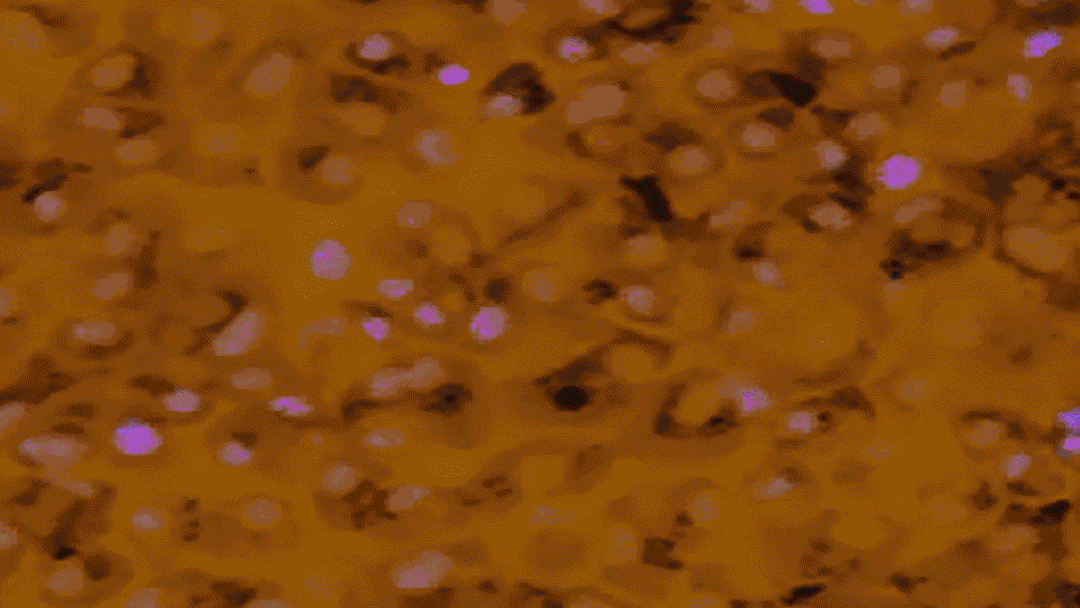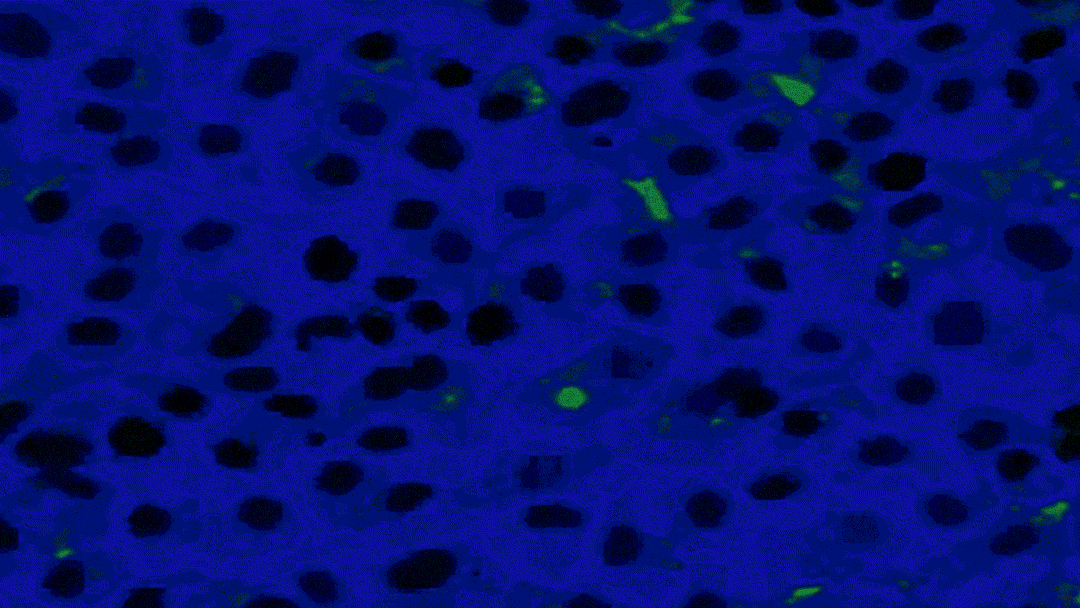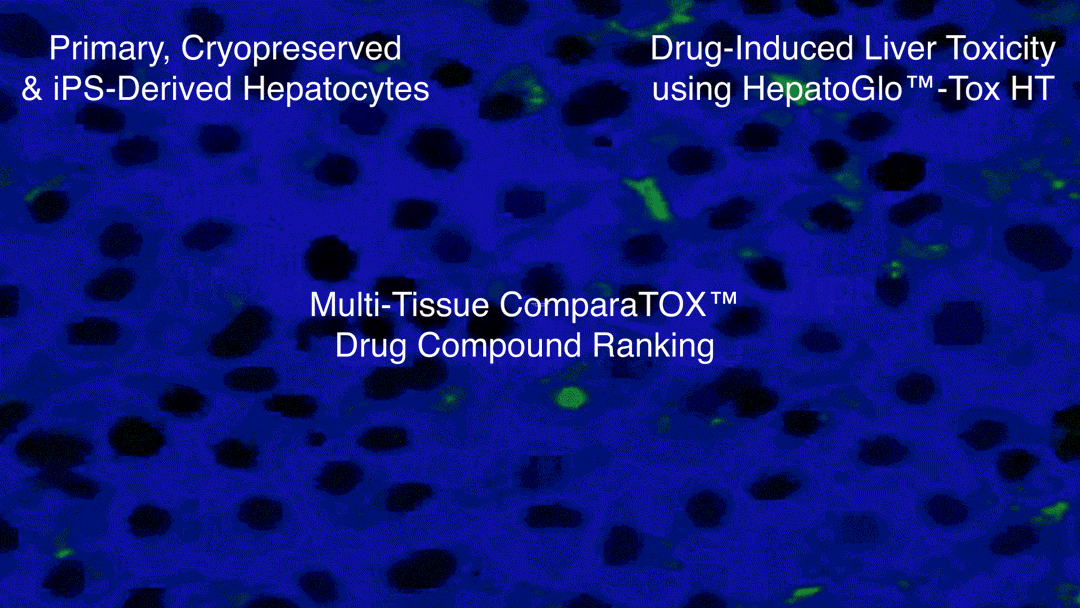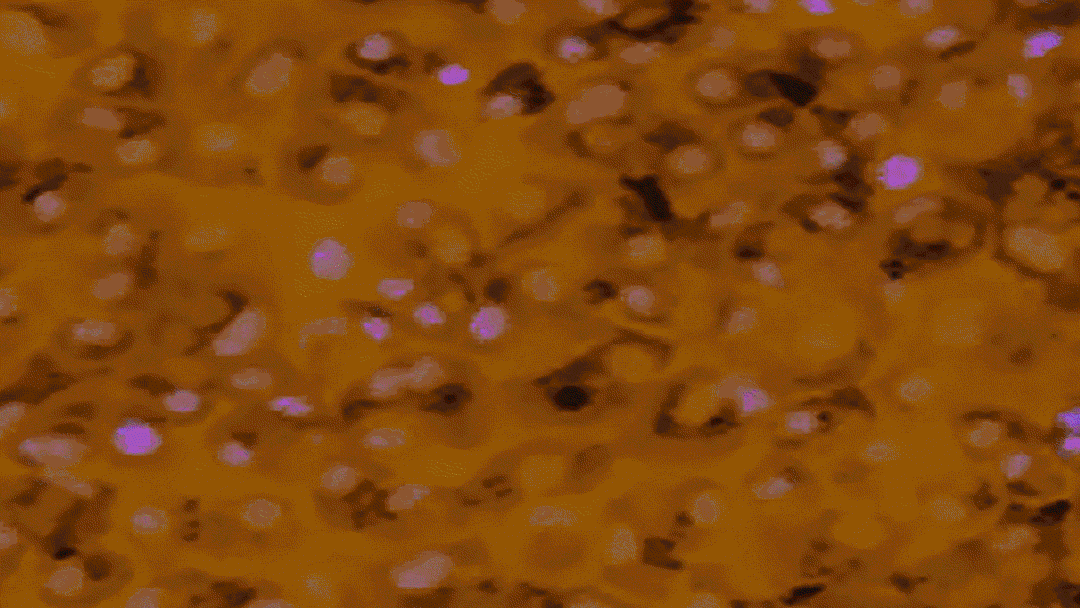
- iCell® Hepatocytes (iPS-derived hepatocytes) from FujiFilm Cellular Dynamics, Inc, used for High-Throughput Hepatotoxicity Screening, Testing and ComparaTOX™ HT
- Fresh
- Cryopreserved
- Cryopreserved, mixed, pooled
- Male
- Female
- Individual or pooled
- Hepatocyte cell lines e.g. HepG2
Species Available for Use With HepatoGlo™-Tox HT
Hepatocytes, usually cryopreserved, are available for the species below. Please contact HemoGenix® for more information.
- Human
- Non-human primate
- Dog
- Rat
- Mouse
Hepatotoxicity Testing: Drug-Induced Liver Toxicity Testing using HepatoGlo™-Tox HT
HepatoGlo™-Tox HT is a proprietary hepatotoxicity testing platform based on an ATP bioluminescence readout (Promega CellTiter-Glo®). It is fully standardized and validated according to FDA Bioanalytical Method Validation Guidelines. For all hepatotoxicity testing and screening, pre-calibration and standardiztion parameters must be compliant with measurement assurance parameters prior to sample processing and measurement.
Since hepatocytes exhibit high metabolic activity, they contain high levels of intracellular ATP (iATP), which can be used to monitor functional viability and, therefore, potential cytotoxicity.
In addition to the characteristics of HepatoGlo™-Tox HT described above, the Platform has the following benefits:
- Available for both 96- and 384-well growth surface-treated, high-throughput testing and screening (see below).
- HepatoGlo™-Tox HT can be used with different oxygen tensions that simulate different conditions and areas in the liver.
- HepatoGlo™-Tox HT is used to investigate drug-drug interactions or multiplexed with CYP450 studies at the sub-cellular level.
- Incubation times: Varies depending on the type of hepatocytes used.
- Turnaround time: Usually within 7 days.
- Validated assay readout according to FDA Bioanalytical Method Guidelines.
- A 3Rs (Reduction, Refinement, Replacement) assay platform for animal testing.
HepatoGlo™-Tox HT High-Throughput Screening and the ComparaTOX™ Platform
As will all other HemoGenix® proprietary toxicity assays, HepatoGlo™-Tox HT was designed for high-throughput screening.
The High-Throughput Screening Module is a rapid, cost-effective service for screening multiples of 5 compounds using either iPS-derived human hepatocytes or cryopreserved, mixed, pooled human hepatocytes.
This service is also part of the HemoGenix® ComparaTOX™ HT Platform in which screening is performed not only on multiples of 5 compounds, but on difference cell types and tissues, including hepatocytes. For example, hepatoxicity might be combined with cardiotoxicity, nephrotoxicity and immunotoxicity. Results are only provided in a single Excel Workbook.
ComparaTOX™ FS is similar to ComparaTOX™ HT, but is not performed in multiples of 5 compounds and includes a full report.
Examples of Hepatotoxicity using HepatoGlo™-Tox HT
Using Google Chrome browser? Right click on image icon. Then click "Open Image in New Tab"
Other Assays for Hepatotoxicity and Hepatocyte Function
Besides HepatoGlo™-Tox HT, the following primary hepatocyte assays are also available:
- Cytochrome P450 enzyme anaysis.
- Membrane integrity assays: LDH or PI dye exclusion.
- Apoptosis: Biochemical caspase detection.
- Mitochondrial dysfunction: Mitochondrial ToxGlo™.
- Glutathione Assay (GSH): Oxidative stress.
- OxyFLOW™: Oxidative DNA damage
Please contact HemoGenix® if an assay is not included, but might be required for a study.
One of the primary questions raised during hepatotoxicity testing is: Do the results from CYP450 studies predict the same results at the cellular level? The CYP450 studies indicate whether a drug inhibits or induces certain cytochrome P450 enzymes resulting in an interaction that might need to be avoided since the consequences could be dangerous.
However, little work has been performed that illustrates any correlation between CYP450 and drug-drug cellular interactions. For this reason, HepatoGlo™-Tox HT is unquiely position to study cellular drug-drug combinations and interactions that might provide more insight into potential hepatoxicity.
Hepatotoxicity Testing and Hepatocyte Functional Assays for In-House Studies
HepatoGlo™-Tox HT and other hepatocyte functional assays are available from Preferred Cell Systems™ for in-house studies. These assays include:
HepatoGlo™-Tox HT for toxicity testing. Please enquire about 384-well plate assays.
HepatoGlo™: Hepatocyte functional assay using an ATP bioluminescence readout.
HepatoFluor™: Hepatocyte functional assay using a non-lytic Resazurin/Resorufin fluorescence readout.
HepatoLight™: Hepatocyte functional assay using a non-lytic tetrazolium WST absorbance readout.
